Helping to Change the Future Through Clinical Research Today
Currently Enrolling Studies:
Welcome to International Clinical Research
IC Research is a dedicated clinical research site company in Sanford, Florida (conveniently located 30 minutes northeast of Orlando) that conducts Phase I-IV clinical research studies in a variety of therapeutic areas.
Featured Study
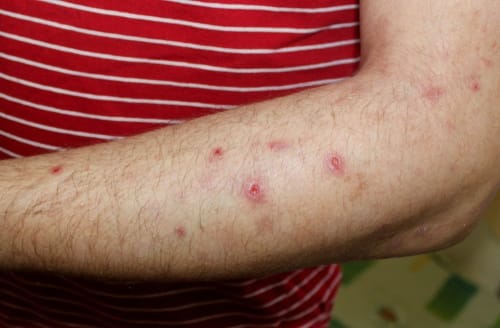
Prurigo Nodularis
We are actively seeking male and female participants aged 18 to 65 who have been diagnosed with Purigo Nodularis (PN). Apply now to be a part of our study.
Knowledge Corner

The Facts About Atopic Dermatitis
February 12, 2024
Atopic Dermatitis, which is a severe form of Eczema, is a chronic and relapsing disease believed to be related to an over-reactive immune system. It

What is Psoriasis?
February 12, 2024
Psoriasis is a chronic and relapsing disease that affects roughly 7.5 million Americans. It is characterized by red patches of raised and scaly skin covered

How Does Endometriosis Affect Women?
October 1, 2019
Endometriosis is a common painful condition that affects women. The tissue that lines the inner cavity of the woman’s uterus (womb) is called endometrium. Endometriosis
Want to Get Involved?
Tell us about yourself and the studies that interest you. We will follow up regarding current and future studies that may be the right fit.